Heat capacity of the nitrogen contact layer on graphite as a function... | Download Scientific Diagram

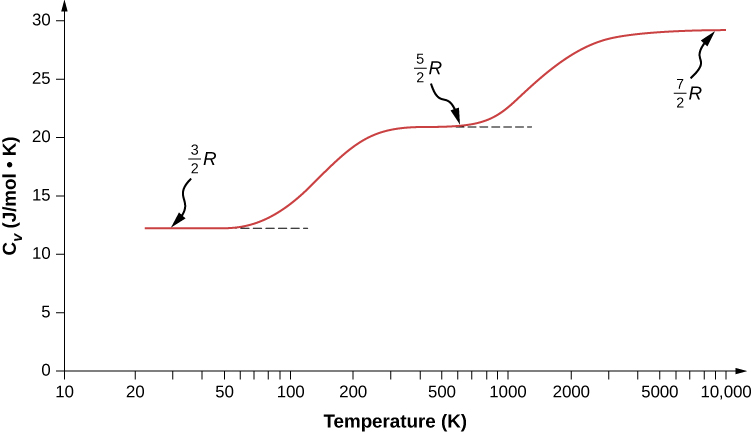
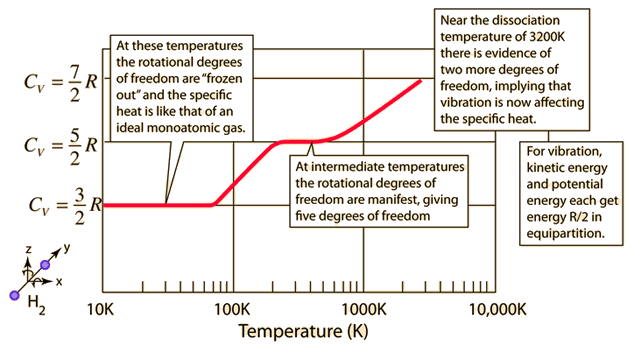
The molar heat capacity at constant pressure of nitrogen gas at `STP` is nearly `3.5 R`.Now when the - YouTube
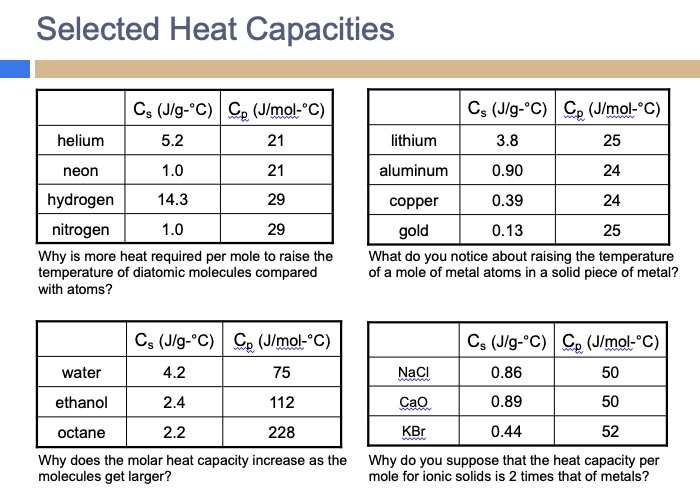
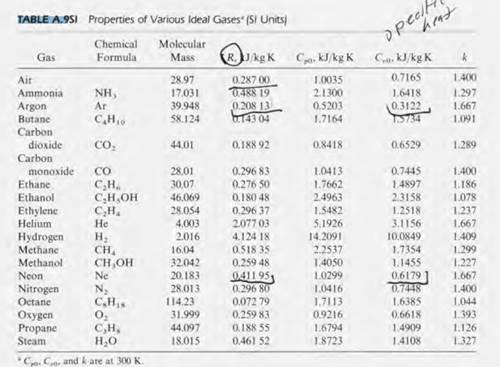
SOLVED: Selected Heat Capacities Cs (Jlg-"C) Cp (Jlmol:"C) helium 5.2 Cs (Jlg-"C) Ce (Jlmol-"C) lithium 3.8 25 neon 1.0 aluminum 0.90 24 hydrogen 14.3 29 29 copper 0.39 24 nitrogen 1.0 Why

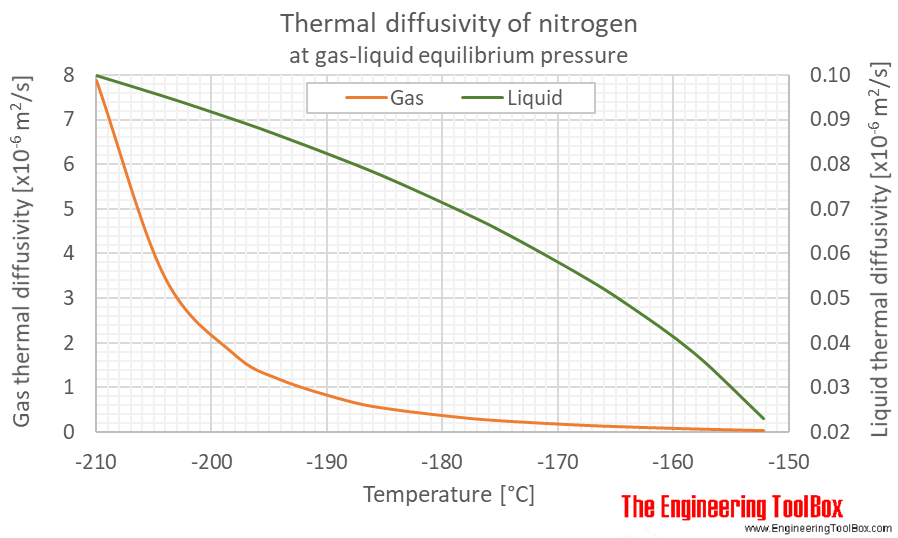
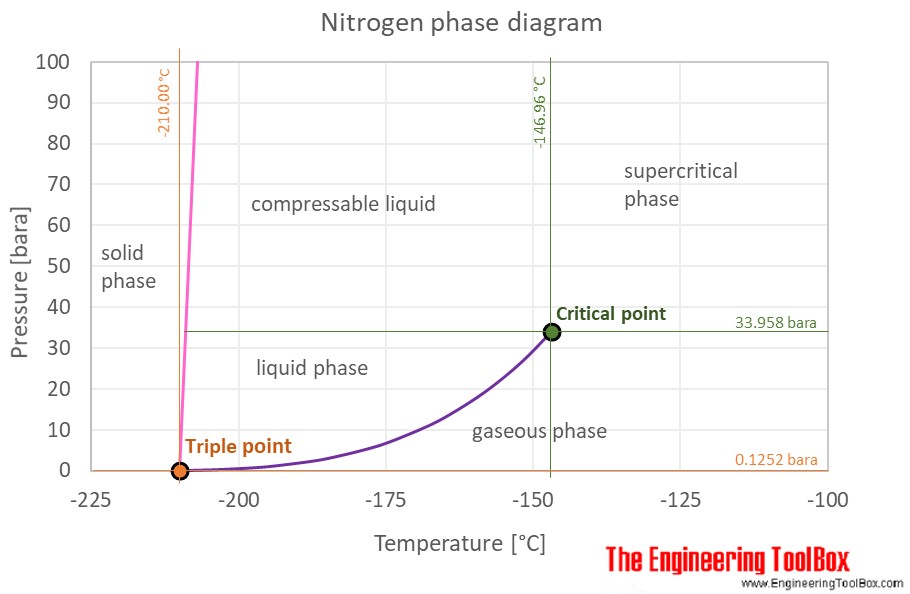
Density and specific heat of nitrogen as function of temperature. Data... | Download Scientific Diagram

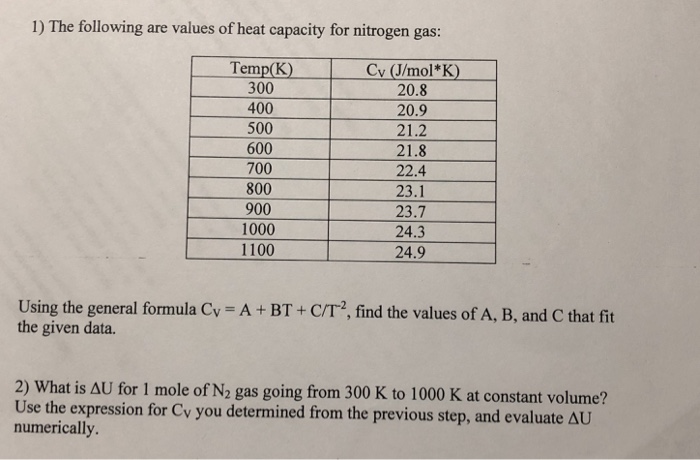
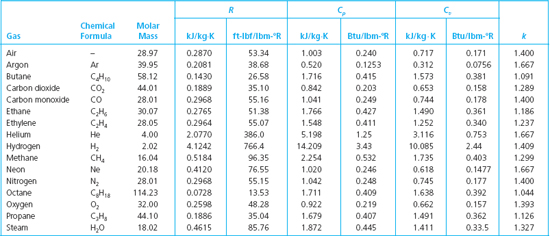
Table 1 from Molar Heat Capacity (Cv) for Saturated and Compressed Liquid and Vapor Nitrogen from 65 to 300 K at Pressures to 35 MPa | Semantic Scholar